Redox titration graph
It is a technique used to characterize and measure the potential of an analyte. For example we can use potassium dichromate to titrate a solution of iron II chloride.

Equivalence Points For Redox Titrations Image And Video Exchange Forumimage And Video Exchange Forum
In this section we demonstrate a simple method for sketching a redox titration curve.

. As in acidbase titrations the endpoint of a redox titration is. The relative positions of the inflection points and equivalence point of a homogeneous redox reaction have been studied by using the redox buffer capacity to derive an equation for the. In this section we demonstrate a simple method for sketching a redox titration curve.
Ce 4 2 Fe 3 Ce 3 Fe Properties of Umass Boston Position of the end point Properties of. 4 different types of potentiometric titrations are known. The titration curve is a drawn by taking the value of this potential E.
Electrode potentials dependent upon dilution I 3 2e-. N x O 32- - so that x 3 -2 -1. Redox titration refers to a laboratory method to determine the analyte concentration by carrying out a redox reaction between the analyte and the titrant.
Oxygen has an oxidation number of -2 and if we use x to represent the oxidation number of nitrogen then the nitrate ion can be written as. In an acid-base titration or a complexation titration the titration curve shows how the concentration. Effect of system variables on redox titration curves Concentration independent of analyte and reagent concentrations.
Potentiometry is also known as Potentiometric titration. The redox titration often needs a. In redox titrations an oxidizing agent is titrated with a reducing agent or vice versa.
Effect of system variables on redox titration curves Concentration independent of analyte and reagent concentrations. REDOX TITRATION CURVE -Redox titration is monitored by observing the change of a electrode potential. Calculate the F from the titration of 1300 mL of 0120 M KF with 1500.
After the equivalence point has been reached there is an excess of the titrant. Redox titration is based on the redox reaction oxidation-reduction between analyte and titrant. Not all titrations require an external indicator.
Our goal is to sketch the titration curve quickly using as few calculations as possible. Redox titration determines the concentration of an unknown solution analyte that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve.
Electrode potentials dependent upon dilution I 3 2e-3 I. Our goal is to sketch the titration curve quickly using as few calculations as possible. A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction.
Titration of a weak base with a strong acid continued Titration curves and acid-base indicators. Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a.
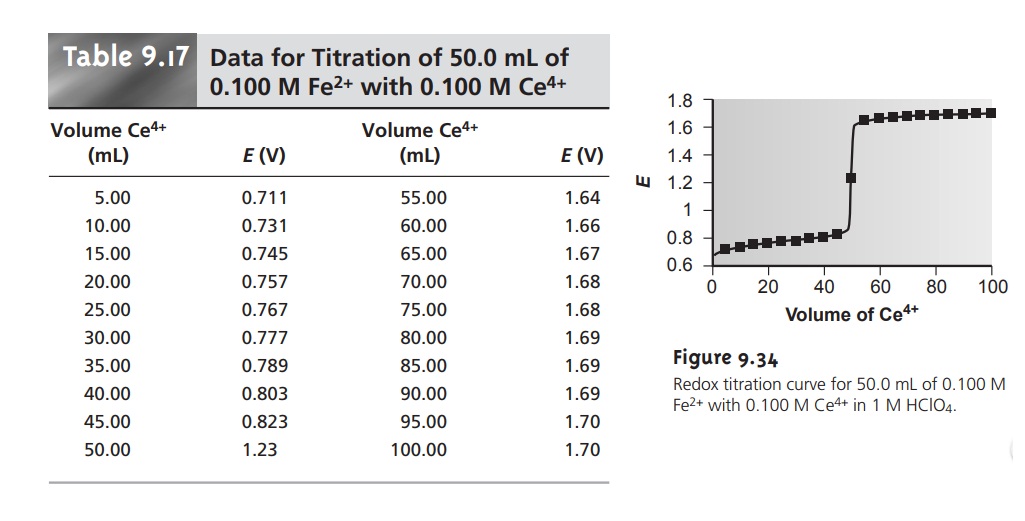
Redox Titration Curves

Fig S5 Redox Titration Curves Of Fluorescence Yield Uncorrected For Download Scientific Diagram

A Redox Titration Curve Of Ferrous Ions In Solution During Reaction Of Download Scientific Diagram

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
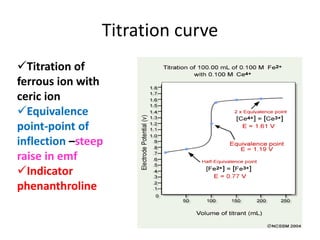
Redox Titration
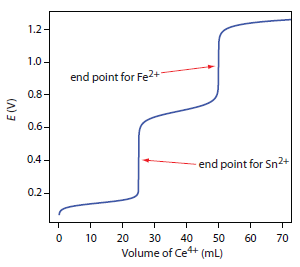
9 4 Redox Titrations Chemistry Libretexts

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
2

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
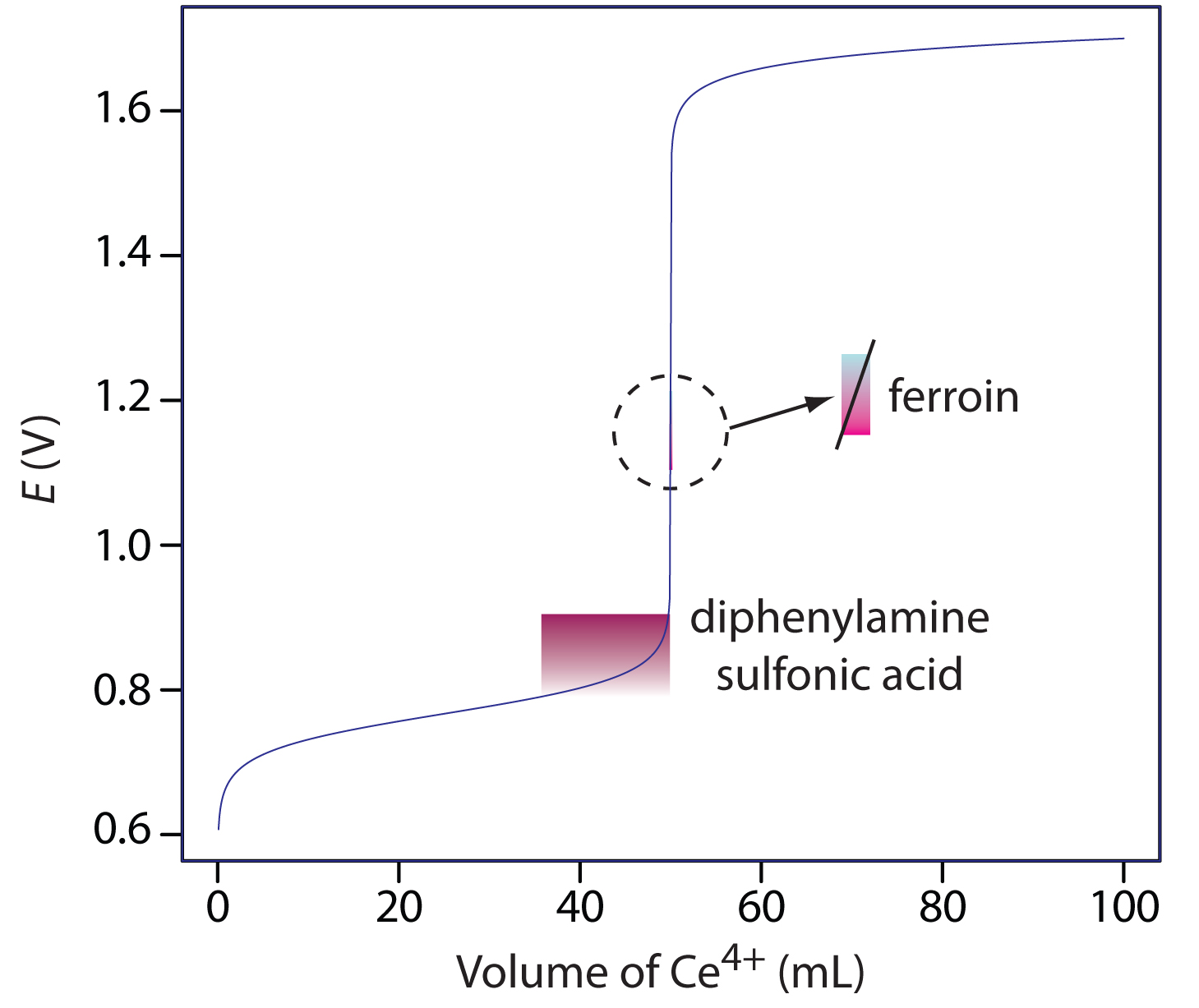
11 4 Reaction Stoichiometry In Solutions Oxidation Reduction Titrations Chemistry Libretexts
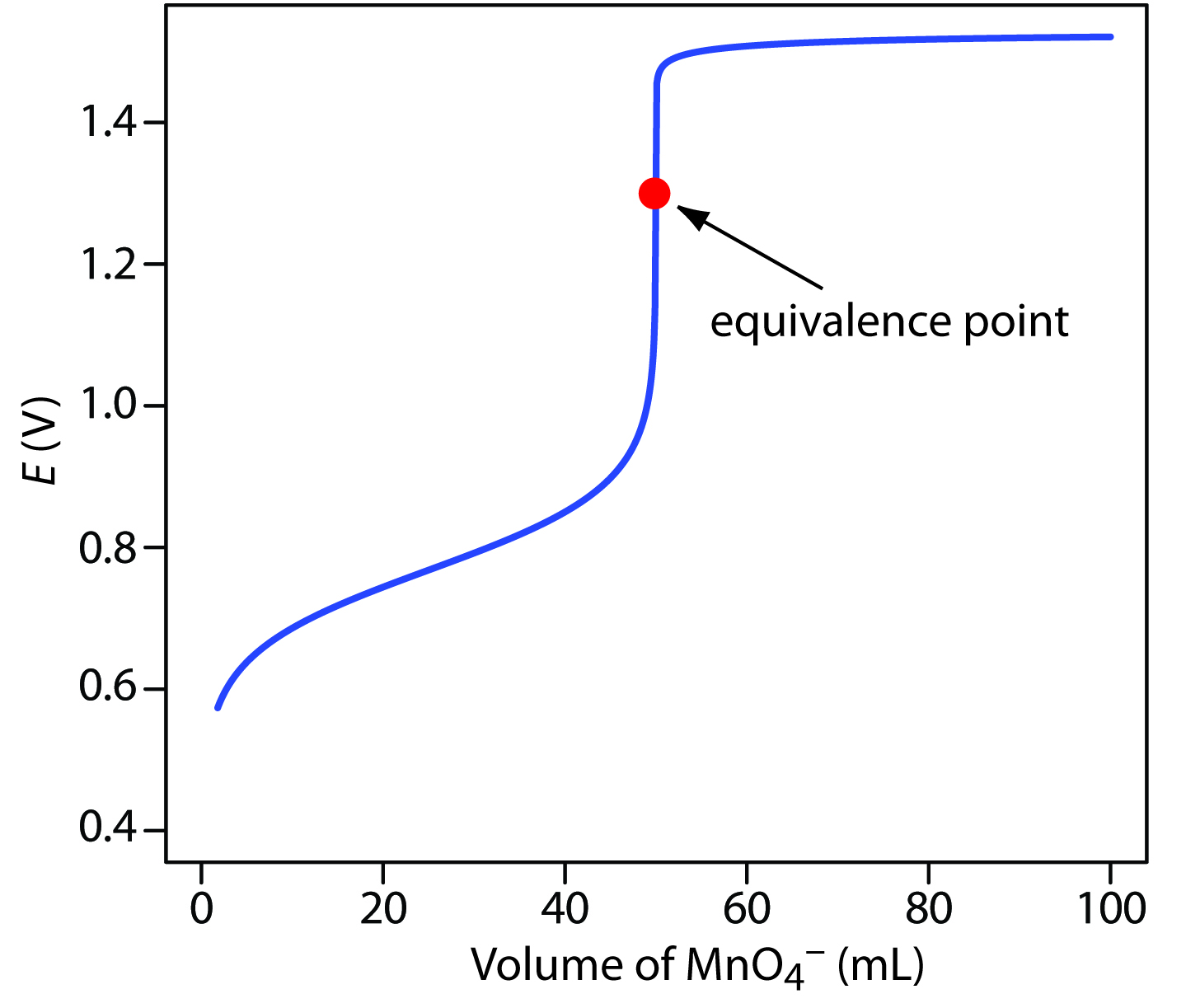
9 4 Redox Titrations Chemistry Libretexts
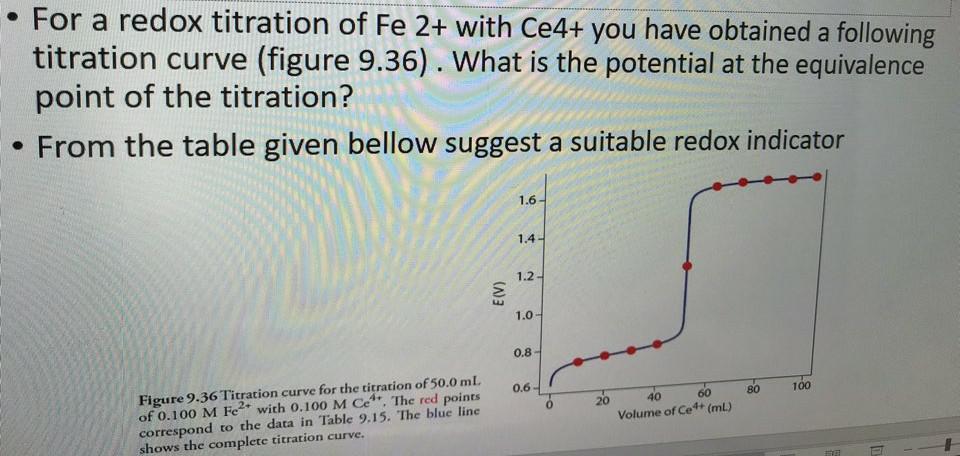
Solved For A Redox Titration Of Fe 2 With Ce4 You Have Chegg Com

9 4 Redox Titrations Chemistry Libretexts
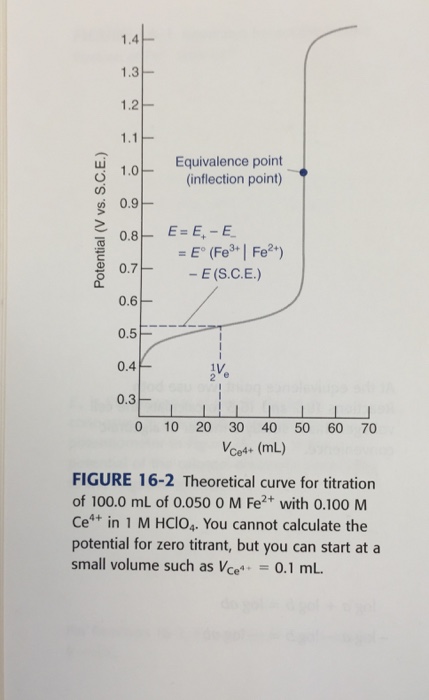
Solved Problems Shape Of A Redox Titration Curve 16 1 Chegg Com

Redox Titration Curves Of Q A In Purified Psii Particles Measured As Download Scientific Diagram

Redox Titration Of The Cofactors Of Nar1 A Titration Curve Of The Download Scientific Diagram
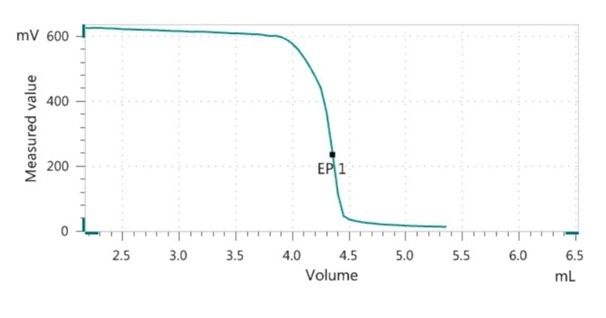
Redox Titration Of Vitamin C Of Milk Powder